Tempo Smart Button™ Intended Use
The Smart Button is intended to detect, store, and transfer insulin dose-related data from a Tempo Pen to a compatible application (App). The Smart Button is indicated for single-patient use by patients 18 years or older who are diagnosed with type 1 or type 2 diabetes mellitus, using prefilled insulin Tempo Pens, and using a compatible App.
TempoSmart and Tempo Insights Indications for Use and Contraindications
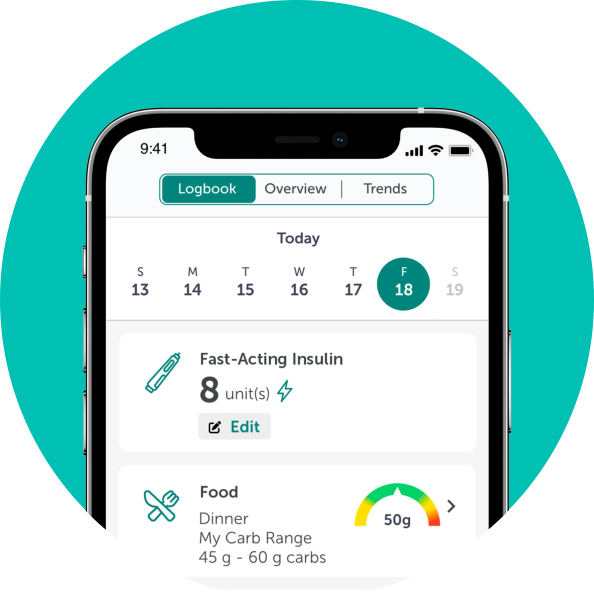
TempoSmart™ is indicated for use by healthcare providers (HCPs) and their patients - aged 18 years and older - who have type 1 or type 2 diabetes. TempoSmart is intended to provide secure capture, storage, and transmission of glucose data as well as information to aid in diabetes self-management. TempoSmart automatically receives insulin dose-related data when connected to a compatible Tempo Smart Button™ device via wireless Bluetooth® technology and has the ability to detect and mark which doses are prime and which are injected insulin. TempoSmart analyzes and reports glucose test results and supports medication adherence. In addition, TempoSmart provides coaching messages (motivational, behavioral, and educational) based on real-time glucose values and trends. It includes software intended for patient use on mobile phones and software intended for healthcare provider use through computer web browsers. The software also allows for entry of other diabetes-related healthcare information and provides educational information.
The following TempoSmart features require a prescription:
For bolus insulin users with type 1 and type 2 diabetes, TempoSmart includes an Insulin Dose Calculator (IDC) to allow patients to use their prescribed regimen to calculate a dose of bolus insulin for a given amount of carbohydrates and/or glucose value from a blood glucose meter (BGM).
When connected to a compatible FDA-cleared integrated continuous glucose monitor (iCGM) and if an insulin-to-carb ratio plus correction factor insulin regimen is prescribed, TempoSmart includes a CGM IDC. The TempoSmart CGM IDC is software intended for the management of type 1 or type 2 diabetes in persons aged 18 years and older requiring fast-acting insulin. The TempoSmart CGM IDC allows patients to calculate a dose of bolus insulin for a given amount of carbohydrates, the most recent CGM glucose reading and rate of change, activity, and insulin on board (IOB).
For basal insulin users with type 2 diabetes, TempoSmart includes a basal titration feature which calculates appropriate long-acting basal insulin doses for titrating insulin levels based on configuration by a healthcare provider. The healthcare provider must activate the basal titration feature and configure it for patient-specific parameters.
Tempo Insights™ is the healthcare provider-facing component of TempoSmart. Neither Tempo Insights nor TempoSmart is intended to replace the research, expertise, judgment, or treatment provided to patients by healthcare providers.
TempoSmart is not indicated for people with gestational diabetes or who use an insulin pump.
Important Safety Information for Tempo™ Personalized Diabetes Management Platform
When using the Tempo Personalized Diabetes Management Platform, if your patient is not sure if they injected their insulin or if their insulin dosing information is accurate, they should NOT start over or repeat the injection. Your patient should monitor their blood glucose per your instructions. Ensure your patient has a backup diabetes management plan if their Tempo Smart Button or TempoSmart App stops working. Having a backup plan and supplies can help avoid hyperglycemia and hypoglycemia. Using the Bolus Insulin Dose Calculator with an incorrect dosing regimen or without all the patient's recent insulin logged may result in unsafe recommendations, which could lead to severe hyperglycemia, severe hypoglycemia, or injury. Carefully review insulin dosing regimen before authorizing the Bolus Insulin Calculator. Keep the Tempo Smart Button away from children. For additional product and safety information, including Warnings and Cautions, consult the Tempo Smart Button Instructions for Use, TempoSmart App User Guides and Tempo Welcome Kit and Tempo Refill Kit Instructions.
Lyumjev® (insulin lispro-aabc) injection 100 units/mL
Indication
Lyumjev is a rapid-acting human insulin analog indicated to improve glycemic control in adults and pediatric patients with diabetes mellitus.
Humalog® (insulin lispro) injection 100 units/mL
Indication
Humalog is a rapid-acting human insulin analog indicated to improve glycemic control in adults and pediatric patients with diabetes mellitus.
Important Safety Information for Lyumjev and Humalog
- Contraindications - Lyumjev and Humalog are contraindicated during episodes of hypoglycemia and in patients who are hypersensitive to these insulins or any of their excipients.
- Warnings and Precautions
Never Share a Lyumjev or Humalog Prefilled Pen, Cartridge, Syringe, or Humalog Reusable Pen Compatible with Lilly 3 mL Cartridges Between Patients, even if the needle is changed. Patients using Lyumjev or Humalog vials must never share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens.
Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen: Changes in insulin strength, manufacturer, type, injection site, or method of administration may affect glycemic control and predispose to hypoglycemia or hyperglycemia. Use caution and close medical supervision when making any changes in insulin regimen and increase the frequency of blood glucose monitoring. Due to reports of hyperglycemia and hypoglycemia, advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor blood glucose. For patients with type 2 diabetes, dosage adjustments of concomitant antidiabetic products may be needed.
Hypoglycemia: Severe hypoglycemia may be life threatening, may lead to unconsciousness, and can cause seizures or death. Hypoglycemia is the most common adverse reaction of Lyumjev and Humalog. Monitor blood glucose and increase monitoring frequency with changes to insulin dosage, coadministered medications, meal pattern, or physical activity; in patients with renal or hepatic impairment; and in patients with hypoglycemia unawareness.
Hypoglycemia Due to Medication Errors: Instruct patients to always check the insulin label before each injection to avoid medication errors. Do not transfer Lyumjev U-200 from the Lyumjev KwikPen® to a syringe and do not transfer Humalog U-200 from the Humalog KwikPen® to a syringe as overdose and severe hypoglycemia can occur.
Hypokalemia: Hypokalemia may be life threatening. Insulins, including Lyumjev and Humalog, cause a shift in potassium from the extracellular to intracellular space possibly leading to hypokalemia, which, if untreated, may result in respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia (e.g., patients using potassium-lowering medications or medications sensitive to serum potassium concentrations).
Hypersensitivity Reactions: Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with Lyumjev and Humalog. If hypersensitivity reactions occur, discontinue the use of Lyumjev or Humalog and treat per standard of care until signs and symptoms resolve.
Fluid Retention and Heart Failure with Concomitant Use of PPAR-gamma Agonists: Thiazolidinediones (TZDs), which are PPAR-gamma agonists, can cause dose-related fluid retention, particularly when used in combination with insulin, including Lyumjev and Humalog. This may lead to or exacerbate heart failure. Observe patients for signs and symptoms of heart failure and consider discontinuation or dose reduction of the PPAR-gamma agonist.
Hyperglycemia and Ketoacidosis Due to Insulin Pump Device Malfunction: Pump or infusion set malfunctions and insulin degradation can lead to a rapid onset of hyperglycemia and ketoacidosis. Prompt identification is necessary. Patients using subcutaneous insulin infusion pumps must be trained to administer insulin by injection and have alternate insulin therapy available in case of pump failure. - Adverse Reactions
Adverse reactions associated with Lyumjev and Humalog include hypoglycemia, hypokalemia, allergic reactions, injection- or infusion-site reactions, lipodystrophy, localized cutaneous amyloidosis, pruritus, rash, weight gain, and peripheral edema. - Drug Interactions
Some medications may alter glucose metabolism, insulin requirements, and the risk for hypoglycemia or hyperglycemia. Signs of hypoglycemia may be reduced or absent in patients taking anti-adrenergic drugs. Particularly close monitoring may be required. - Pediatric Use
Lyumjev-treated pediatric patients reported a higher incidence of subcutaneous injection site-related reactions compared to Lyumjev-treated adults. It is expected that Lyumjev-treated pediatric patients who receive continuous subcutaneous insulin infusion (CSII) are more likely to have infusion site-related adverse reactions than those who receive subcutaneous injections. Monitor injection and infusion sites closely when initiating therapy with Lyumjev in pediatric patients. If persistent injection or infusion site reactions occur, discontinue Lyumjev and initiate therapy with an alternative insulin.
UR HI HCP ISI 14OCT2022
Please click to access Lyumjev Full Prescribing Information including Patient Information and Instructions for Use for the Lyumjev® Tempo Pen™.
Please click to access Humalog U-100 Full Prescribing Information including Humalog U-100 Patient Information and Instructions for Use for the Humalog® Tempo Pen™.
BASAGLAR® (insulin glargine) injection 100 units/mL
Indication
BASAGLAR® is a long-acting human insulin analog indicated to improve glycemic control in adults and pediatric patients with type 1 diabetes mellitus and in adults with type 2 diabetes mellitus.
Limitations of Use:
BASAGLAR is not recommended for the treatment of diabetic ketoacidosis.
Important Safety Information for BASAGLAR
Contraindications - BASAGLAR is contraindicated during episodes of hypoglycemia, and in patients with hypersensitivity to insulin glargine or any of the excipients in BASAGLAR.
Warnings and Precautions
BASAGLAR prefilled pens must never be shared between patients, even if the needle is changed. Sharing poses a risk of transmission of blood borne pathogens.
Changes in insulin strength, manufacturer, type, injection site, or method of administration may affect glycemic control and predispose to hypoglycemia or hyperglycemia. Any changes in insulin regimen should be made cautiously and only under close medical supervision, and the frequency of blood glucose monitoring should be increased. Due to reports of hypoglycemia and hyperglycemia, advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor blood glucose. For patients with type 2 diabetes, dosage adjustments of concomitant anti-diabetic products may be needed.
Hypoglycemia is the most common adverse reaction associated with insulins, including BASAGLAR. Severe hypoglycemia can cause seizures, may be life-threatening, or cause death.
Accidental mix-ups between insulin products have been reported. To avoid hypoglycemia due to medication errors between BASAGLAR and other insulins, instruct patients to always check the insulin label before each injection.
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulins, including BASAGLAR. If hypersensitivity reactions occur, discontinue BASAGLAR; treat per standard of care and monitor until symptoms and signs resolve. BASAGLAR is contraindicated in patients who have had hypersensitivity reactions to insulin glargine or one of the excipients.
All insulins, including BASAGLAR, cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia if indicated.
Thiazolidinediones (TZDs), which are peroxisome proliferator-activated receptor (PPAR)-gamma agonists, can cause dose-related fluid retention, particularly when used in combination with insulin. Fluid retention may lead to or exacerbate heart failure. These patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of TZD must be considered.
Adverse Reactions
Adverse reactions commonly (>5%) associated with insulin glargine products are: hypoglycemia, allergic reactions, injection site reaction, lipodystrophy, pruritus, rash, edema, and weight gain.
Drug Interactions
Certain drugs may affect glucose metabolism, requiring insulin dose adjustment and close monitoring of blood glucose.
The risk of hypoglycemia may increase when antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analog (e.g., octreotide), and sulfonamide antibiotics are co-administered with BASAGLAR.
The blood glucose lowering effect of BASAGLAR may decrease when co-administered with atypical antipsychotics, corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones. The blood glucose lowering effect of BASAGLAR may increase or decrease when co-administered with alcohol, beta-blockers, clonidine, lithium salts, and pentamidine.
The signs and symptoms of hypoglycemia may be blunted when beta-blockers, clonidine, guanethidine, and reserpine are co-administered with BASAGLAR.
BV HCP ISI 14SEP2022
For more information, please click to access Full Prescribing Information including Patient Information and Instructions for Use for the BASAGLAR Tempo Pen.